 |
Pacemaker Follow-up
|
|||||||||
 FEATURES:
BENEFITS:
|
||||||||||
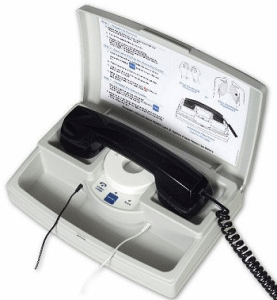
| The PSD 410 Pacemaker Transmitter allows for the easiest, most reliable and accurate follow-up of pacemaker patients. The PSD 410 was designed with leading edge technology to digitize the incoming signal and translate it to a frequency synthesized output. This will result in a clear electrocardiogram with easy to read, identically consistent pacer enhancements. The PSD 410 Pacemaker Transmitter offers improved patient compliance with an advanced patient alert feature, one-button operation, and clear instructions. The PSD 410 comes with a test magnet, lead wires, and your choice of skin or adjustable wrist electrodes. The unit carries all of its accessories in one convenient case, and offers the option for telephone touch-tone control or a reversible telephone cradle. |
||||||||||
PHYSICAL SPECIFICATIONS:
TRANSMISSION CHARACTERISTICS:
ELECTRICAL SPECIFICATIONS:
COMMUNICATIONS OPTIONS:
|
||||||||||
|
Integrated Medical Devices, Inc.
549 Electronics Parkway Liverpool, New York 13088 USA Tel: 315.885.6400 www.imdinc.com sales@imdinc.com © Integrated Medical Devices, Inc. 2007 |
||||||||||